Common Adverse Reactions of Influenza Vaccine and Countermeasures
Original Guo Lin Ji Lianmei Ji Lianmei Pharmacist
Everyone should have seen the hot search this morning about the death of 28 people in South Korea after being vaccinated with influenza vaccine, which made many friends worry about the safety of influenza vaccine.
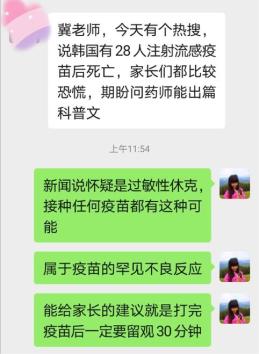
I probably followed the news. At present, I suspect that two cases are related to anaphylactic shock. The relationship between other deaths and influenza vaccine has not been established and is still under investigation.
It is said that the death toll rose to 36 in the afternoon, but South Korea did not stop the flu vaccination. It is likely that the death has nothing to do with the vaccine. This incident is unusual, and I will continue to pay attention to the progress of this incident.
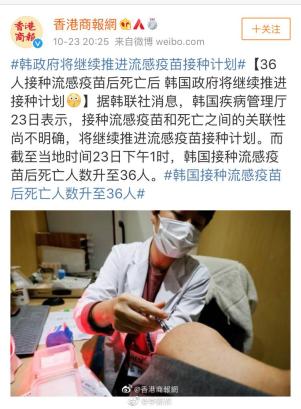
With regard to these two deaths suspected of anaphylactic shock, let me popularize the knowledge of adverse reactions related to influenza vaccine.
Vaccines are medicines, and adverse reactions may occur when any medicine is used. Anaphylactic shock is a rare adverse reaction that may occur when any vaccine is inoculated.
Moreover, whether to treat diseases or prevent diseases, whether to use drugs or not is a choice after repeatedly weighing risks and benefits.
As far as influenza vaccine is concerned, it is a relatively mature drug. If its adverse reactions are weighed against the risk of serious complications after getting the flu, the advantages of influenza vaccination must outweigh the disadvantages. Therefore, mainstream medical views have always recommended that everyone get the flu vaccine, and I personally recommend it, and I have always adhered to the relevant knowledge of popular science influenza vaccine.
There are three types of influenza vaccines this year, namely inactivated trivalent influenza vaccine and tetravalent influenza vaccine by intramuscular injection, and attenuated trivalent influenza vaccine by nasal spray.
Below, we will talk about their common adverse reactions respectively.
First, inactivated influenza vaccine (including trivalent and tetravalent)
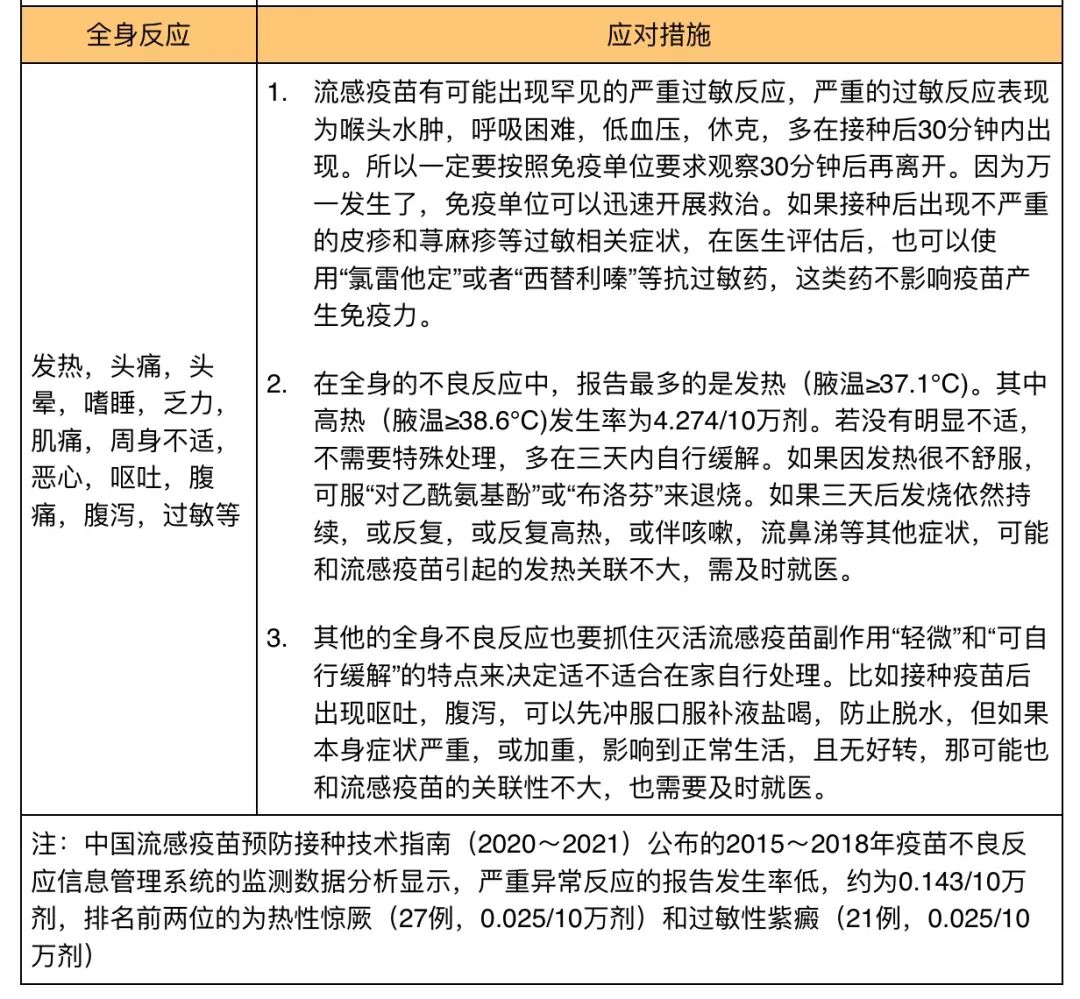
The common side effects of inactivated influenza vaccine by intramuscular injection are mainly manifested in two types:
Local reaction, that is, swelling, induration, pain, burning sensation at the inoculation site, etc.
Systemic reactions, namely fever, headache, dizziness, lethargy, fatigue, myalgia, general malaise, nausea, vomiting, abdominal pain, diarrhea, allergies, etc.
These side effects have two characteristics:
First, it is usually mild, with few severe reactions.
Second, it can fade by itself within a few days.
In order to look more intuitive, we summarize these common side effects and their countermeasures as follows:

Two, freeze-dried nasal spray influenza attenuated trivalent vaccine
In this year (2020), freeze-dried live attenuated nasal spray influenza vaccine was newly launched in China. During the phase III clinical trial of the vaccine, 4,500 subjects aged 3-17 were vaccinated once, and the safety was observed for more than 6 months after vaccination. The results are as follows:
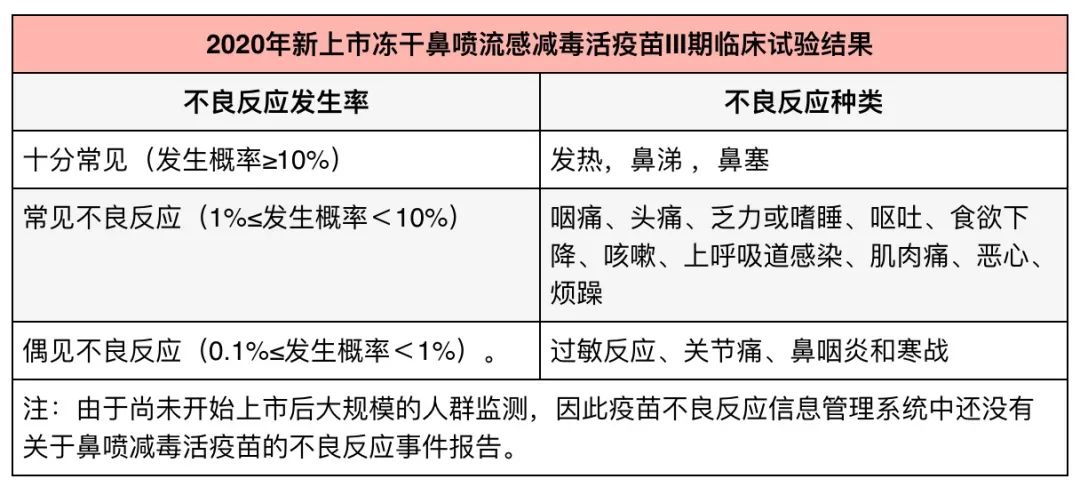
From the data point of view, the overall safety of this vaccine is good. The safety data of similar products abroad are similar, with mild adverse reactions and self-healing. Serious adverse reactions are rare.
However, because the vaccine has just been applied in China this year, the biggest challenge for vaccinators and recipients is unfamiliarity, so both sides need to strictly grasp the contraindications of attenuated nasal spray vaccine, so as to avoid predictable adverse reactions.
For example, although there is a needle in the vaccine box, this needle is used to prepare lyophilized powder for dissolving the vaccine, and the final vaccine is inoculated by "nasal spray". If the vaccinator is negligent and takes it for granted that he can inject with an injection needle, it will bring harm to the vaccinator.
For example, if the vaccinator has rhinitis or asthma, he can’t use the nasal spray live vaccine, because the nasal mucosa congestion and swelling caused by rhinitis may weaken the effect of the vaccine, and the rhinitis that may occur after vaccination may also be confused with the original rhinitis. It is also not suitable for children who are regularly receiving nasal spray treatment.
Another example is that the vaccine is a live vaccine, which is not suitable for children with impaired immune function, because it may theoretically cause children with impaired immune function to contract the flu. However, children with impaired immune function can be safely vaccinated with inactivated influenza vaccine.
After vaccination, you need to observe in the immune unit for 30 minutes to ensure that there is no serious allergic reaction before leaving.
Finally, I would like to reiterate my view on influenza vaccine: it is a relatively mature drug. If we weigh its adverse reactions with the risk of serious complications after getting the flu, the advantages of influenza vaccine must outweigh the disadvantages.
I hope everyone will not give up eating because of choking.
I also recorded a short video of related knowledge, which will be posted on the video number tomorrow. Welcome to identify the QR code below and pay attention to my video number.
If the dry goods explained in the video are helpful to you, please help me share them with more parents. Every praise, forwarding and comment from you is the motivation for me to keep updating the video number. Thank you for your support!
People who like this content also like it.
Original title: Common Adverse Reactions of Influenza Vaccine and Countermeasures
Read the original text